Background: Since the introduction of tyrosine kinase inhibitor therapy survival has substantially improved in CML. However, a subset of patients become resistant and progress to blast phase. Still the underlying genomic changes are not completely understood.
Cohort & Methods: We studied 21 CML patients with paired sample material available from diagnosis in chronic phase (CP) and from blast phase (BP). All patients received a tyrosine kinase inhibitor starting at diagnosis of CML CP. Samples were analyzed by cytomorphology, chromosome banding analysis, WGS (100x, 2x151bp) and WTS (50 Mio reads, 2x101bp). B-cell receptor (BCR) rearrangements were analyzed with MiXCR software. The transcriptomic phenotype (TP) of each CP and BP sample was estimated by mapping their profiles to representative cases diagnosed with B-ALL, T-ALL, MDS, CMML, AML and CML CP.
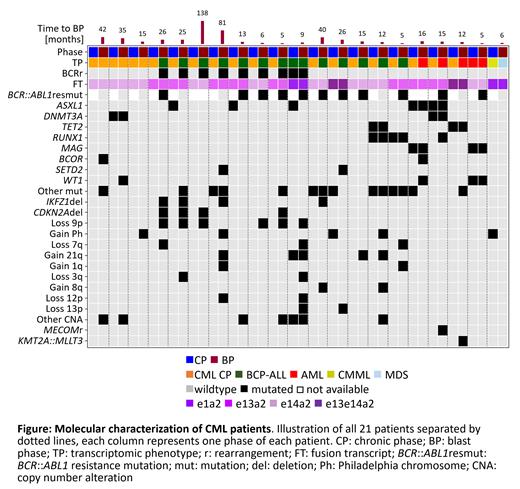
Results: The median time between diagnosis of CP and BP was 14.7 months (range: 4.7 - 137 months). While 18/21 CML in CP showed a TP corresponding to CML CP, one case each showed a TP corresponding to CMML, AML and B-ALL, respectively, although bone marrow blast counts were 6.5%, 5.5% and 5.5%, respectively. Of note, the case with the CMML-TP harbored a p190 BCR:: ABL1 isoform and also resembled CMML in bone marrow morphology. These three cases progressed to BP after 5.8, 5.3, and 8.9 months. The TP in BP corresponded to B-ALL in 13 cases, while 4, 3, and 1 case showed an AML-, CML CP- and MDS-TP, respectively (figure). In CP recurrent genomic alterations in addition to the BCR:: ABL1 fusion were mutations in ASXL1 (n=5), DNMT3A, TET2, RUNX1, and MGA (2 cases each). In BP the genomic landscape was more complex. Additional genetic alterations compared to CP were detected in all cases. Recurrently gained mutations in BP were mutations in WT1, BCOR, and SETD2 (2 cases each). The gain of copy number alterations (CNA) was more frequent. Recurrent CNA were: loss 9p (n=6), gain Ph-chromosome (n=4), loss 7q (n=3), gain 21q (n=3), gain 1q, loss 3q, gain 8q, loss 12p, and loss 13q in 2 patients each. Only three balanced structural variants were gained: a MECOM-, a NUP98:: HHEX- and a KMT2A:: MLLT3-rearrangement. Further, in 11/21 BP resistance mutations within the BCR:: ABL1 kinase domain were detected. The variant allele frequency (VAF) of RUNX1 mutations increased in BP in both cases with RUNX1 mutation present in CP (5% to 42%, time to BP: 4.7 months; 8% to 59%, time to BP: 11.7 months). DNMT3A and MGA mutations were about stable (VAF CP/BP: DNMT3A: 48%/37%, 33%/46%; MGA: 52%/36%, 40%/52%), while TET2 mutations were stable in one case (46%/49%) and decreased in the second (57%/27%). ASXL1 mutations were stable in 2 cases with an AML-TP in BP and lost in 3/5 cases with a B-ALL-TP in BP. BP with a lymphoid TP differed substantially with respect to the genomic profile from BP with myeloid TP. WTS data revealed a clonal BCR rearrangement in 8/13 BP with B-ALL-TP, of which 4 rearrangements are predicted to be productive. Further, CDKN2A/B deletions, IKZF1 deletions, and SETD2 mutations were only found in BP with B-ALL-TP. Thus, CML in BP with a B-ALL-TP showed a genomic landscape similar to B-ALL with BCR:: ABL1-rearrangement. In contrast CML BP cases with an AML-TP (n=4) had gained either rearrangements typically found in AML such as a KMT2A:: MLLT3 rearrangement and a MECOM rearrangement or acquired mutations in RUNX1, BCOR and WT1, or a CN-LOH 11p in combination with a WT1 VAF increase from 51% to 94%. Cases showing still a CML CP-TP (n=3) in BP acquired rather few additional genetic abnormalities (mutations in BCOR, WT1, CTCF; NUP98:: HHEX-rearrangement, gain of Ph-chromosome, loss of 11p, 17q), which were more myeloid in appearance.
Conclusions: 1) Extensive genetic profiling indicated a substantial clonal evolution in the progression from CP to BP CML including loss of ASXL1 mutations, expansion of RUNX1 mutated clones, multiple CNA, and the frequent acquisition of a BCR rearrangement in BP with a transcriptomic phenotype resembling B-ALL. 2) A subset of CML cases in CP already showed a transcriptomic phenotype resembling acute leukemia indicating a rapid progression to BP. 3) The presence of a RUNX1 mutated subclone or a clonal BCR rearrangement seem to represent a warning signal in CML CP. 4) The prognostic validity of an extended genetic profiling including ASXL1, RUNX1, BCR rearrangement, and transcriptional signature in CML CP needs to be assessed in forthcoming prospective studies.
Disclosures
Haferlach:MLL Munich Leukemia Laboratory: Current Employment, Other: Equity Ownership. Walter:MLL Munich Leukemia Laboratory: Current Employment. Mueller:MLL Munich Leukemia Laboratory: Current Employment. Huber:MLL Munich Leukemia Laboratory: Current Employment. Baer:MLL Munich Leukemia Laboratory: Current Employment. Hutter:MLL Munich Leukemia Laboratory: Current Employment. Meggendorfer:MLL Munich Leukemia Laboratory: Current Employment. Hoermann:MLL Munich Leukemia Laboratory: Current Employment. Kern:MLL Munich Leukemia Laboratory: Current Employment, Other: Equity Ownership. Haferlach:MLL Munich Leukemia Laboratory: Current Employment, Other: Equity Ownership.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal